Ammonia Fountain
Purpose:
To demonstrate the solubility of a gas and the effect of a gas pressure difference.
Materials:
- 1-hole rubber stopper to fit Erlenmeyer flask
- 1L boiling/round-bottom flask
- 2-hole rubber stopper to fit boiling/round-bottom flask
- 500mL Erlenmeyer flask
- Large plastic syringe
- Long U-shaped glass tube
- Long rubber hose
- Long straight glass tube
- Ring clamp
- Ring stand
- Short straight glass tube
- 600mL beaker
Reagents:
- Ammonium chloride (NH4Cl(s))
- Sodium hydroxide (NaOH(s))
- Phenolphthalein indicator
Hazards and PPE:
- Ammonium chloride is a toxin and irritant. Handle with care.
- Sodium hydroxide is corrosive and toxic. Handle with care.
- Wear approved safety goggles and gloves.
Protocol:
Preparation
- Demo setup should be as shown:
-
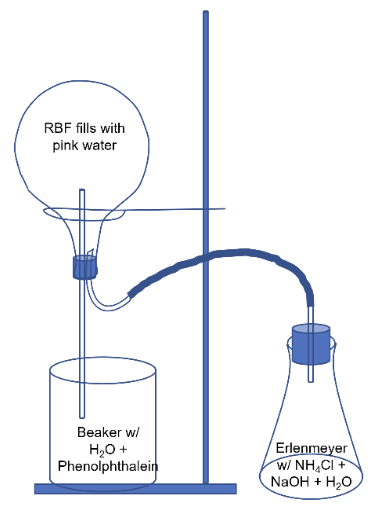
- Fill 600mL beaker with 500mL DI H2O and add 3 drops phenolphthalein indicator.
- Fill large syringe with DI H2O.
- Place long and u-shaped glass tubes into 2-hole rubber stopper as shown.
- Attach rubber hose to u-shaped glass tube. Connect the other end of the hose to the short glass tube that’s in a 1-hole stopper.
- Invert the round-bottom flask and insert the 2-hole stopper assembly suspended high enough that long glass tube is not in the beaker as shown.
- Measure 16g NaOH(s) and 20g NH4Cl(s) in separate weigh boats.
- Right before demo is performed: In the fume hood, add both solids to the Erlenmeyer flask.
- Add 20mL DI H2O to Erlenmeyer flask and immediately stopper flask with the 1-hole rubber stopper.
- Swirl the Erlenmeyer flask to aid in the reaction, causing ammonia gas to fill the RBF.
- After ~20 seconds, disconnect hose from the Erlenmeyer’s glass tube and connect to syringe filled with DI H2O. Immediately move entire setup to demonstration bench.
Demonstration
- Lower round bottom flask so that long glass tube is submerged as far as possible into beaker of water and phenolphthalein.
- To initiate the fountain, use the syringe to inject a small amount of water into the round-bottom flask.
- Water will rise into the tube and will spray into the round-bottom flask as a pink fountain.
Additional Notes:
- Lecture bottles of ammonia gas are difficult to obtain, but can be substituted for the gas generator used here if you have on available.
- Ammonia is flammable and may form explosive mixtures with air. It is harmful if inhaled and can displace oxygen to cause rapid suffocation. Only use it in a fume hood. Ammonia causes severe skin burns and eye damage and is very toxic to aquatic life - never let it go down the drain.
Disposal:
- Solution should be neutralized before being washed down the sink with excess water.
Citations:
- This demo was adapted by me for the demo library of the Chemistry department at the University of Illinois at Urbana-Champaign.

