Different Colored Complexes with Different Numbers of Ligands
Purpose:
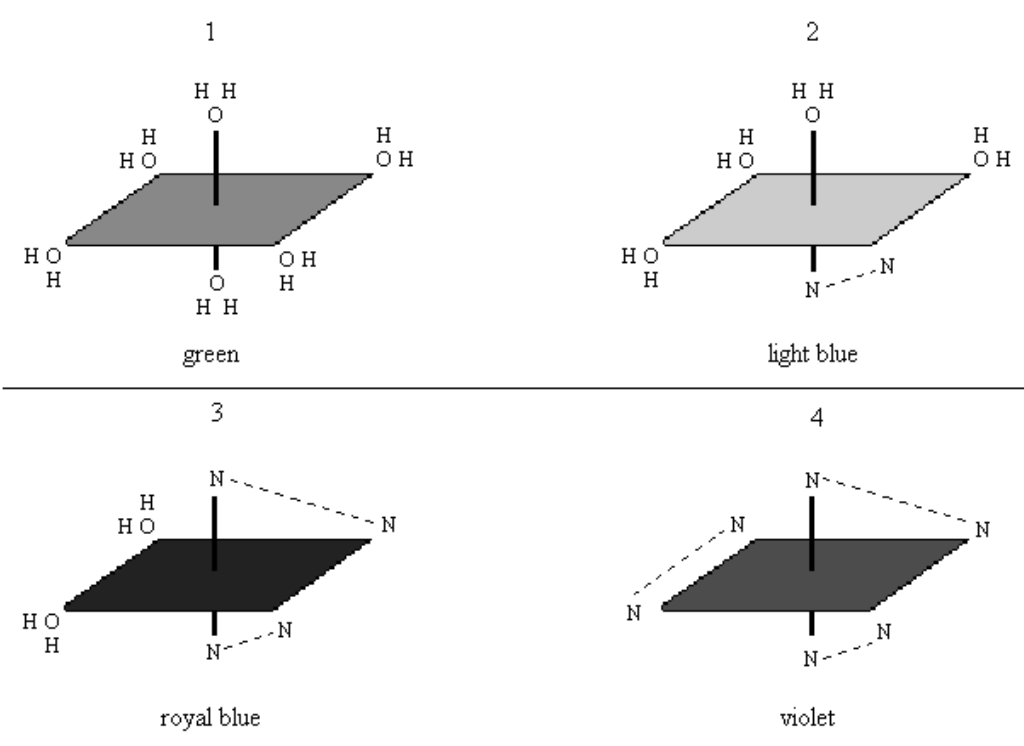
To demonstrate that the number of ligands (the same ligands) can alter the color of coordination complexes.
Materials:
- 50mL beaker
- 4 400mL beakers
Reagents:
- Ethylenediamine (C2H4(NH2)2)
- 1M Nickel chloride (NiCl2)
Hazards and PPE:
- Ethylenediamine is flammable and an acute toxin. Store and handle with care.
- Nickel chloride is an acute toxin, environmental hazard, germ cell mutagen, carcinogen, and reproductive toxin. Handle and dispose of with care.
- Wear approved safety goggles and gloves.
Protocol:
Preparation
- In a fume hood, pour ~10mL ethylenediamine into a small labeled beaker. Cover with parafilm.
- Fill 4 400mL beakers with 280mL DI H2O.
Demonstration
- Add 20mL 1M NiCl2 to all 4 beakers of DI H2O.
- To beaker 2, add ~1mL ethylenediamine. Stir until a light blue color appears; if still green, add drops of ethylenediamine until light blue color appears.
- To beaker 3, add ~2.5mL ethylenediamine. Stir until a royal blue color appears; if still green, add drops of ethylenediamine until royal blue color appears.
- To beaker 4: add ~4mL ethylenediamine. Stir until a violet color appears. If not, add drops of ethylenediamine until violet color appears.
Disposal:
- Waste should be collected in a properly labelled container.
- Rinse all glassware with acetone first, then wash with regular soap and water.
Reactions:
- NiCl2 + 6H2O ⇌ Ni(H2O)62+ + 2Cl-(aq)
- Ni(H2O)62+(aq) + en ⇌ [Ni(H2O)4(en)]2+(aq) + 2H2O(l)
- Ni(H2O)62+(aq) + 2en ⇌ [Ni(H2O)2(en)2]2+(aq) + 4H2O(l)
- Ni(H2O)62+(aq) + 3en ⇌ [Ni(en)3]2+(aq) + 6H2O(l)
-
Citations:
- L.R. Summerlin, C.L. Borgford, and J.B. Ealy, 1987, Chemical Demonstrations: A Source Book for Teachers, vol. 2, pp 73-74.
Videos:

